The Sky Was Falling: The Unlikely Story of How We Saved the Ozone Layer
How a handful of scientists, a strange discovery in Antarctica, and a global treaty healed a hole in our planet's shield.
Something was wrong in Antarctica. For two years, from 1982 to 1984, the readings had been bizarre. The sophisticated instrument at the British Antarctic Survey’s Halley Bay station, a device designed to measure the thickness of the ozone layer, kept spitting out numbers that were not just low, but absurdly, impossibly low. A 40% drop in springtime ozone. It had to be a ghost in the machine. The scientists, led by Joe Farman, did what any rational person would: they assumed their expensive equipment was broken and ordered a new one.¹
For two long, cold seasons, they set the data aside. But when the new instrument arrived and confirmed the terrifying readings of the old one, a chilling realization washed over the team. The instrument wasn't broken. The sky was.
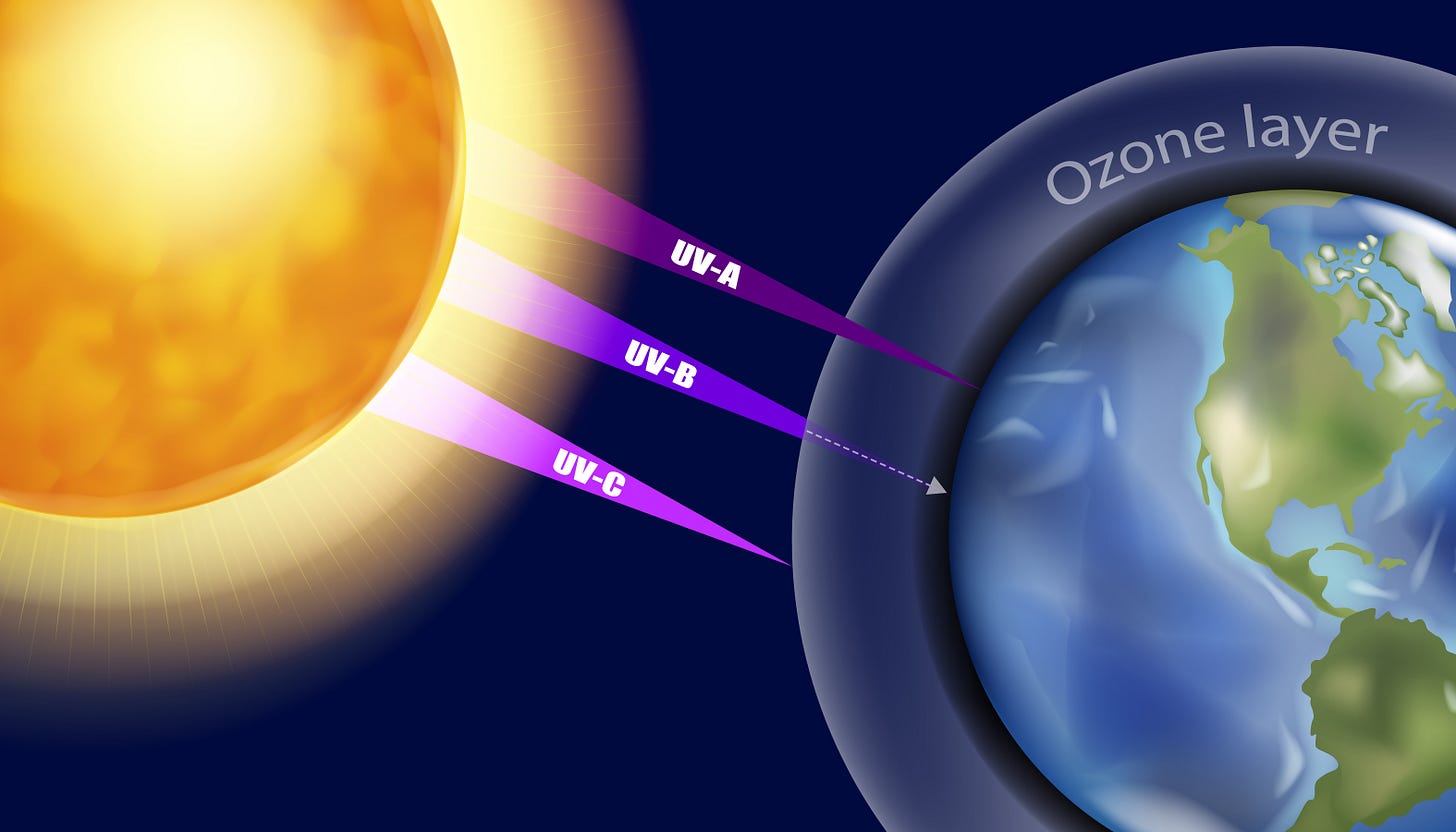
Our planet wears an invisible guardian. High in the stratosphere, some 10 to 30 miles above our heads, floats a delicate concentration of molecules called ozone. Each ozone molecule is a simple trio of oxygen atoms (O₃), bound together in a shape that makes it a master at absorbing the sun’s most aggressive ultraviolet rays, specifically UV-B radiation. This planetary sunscreen is the only reason life as we know it can exist on Earth’s surface. Without it, the sun’s energy would scorch the continents, sterilize the seas, and cause catastrophic rates of skin cancer and cataracts in animals and humans.
We owe our lives to this thin veil of gas. And in the 1980s, we discovered we were systematically destroying it.
The Chemical Detectives
The first whisper of trouble came more than a decade earlier, in a quiet university lab in California. It was 1974, and two chemists, Mario Molina and F. Sherwood “Sherry” Rowland, were investigating a class of popular industrial chemicals: chlorofluorocarbons, or CFCs.
You may know them by their trade name, Freon. In the mid-20th century, CFCs were considered miracle molecules. They were non-toxic, non-flammable, and incredibly stable—perfect as refrigerants in air conditioners, propellants in aerosol cans, and agents for blowing foam insulation. They seemed utterly harmless. But Rowland and Molina’s calculations told a different, more sinister story.²
Their hypothesis was as elegant as it was alarming. Because CFCs were so stable, they didn’t break down in the lower atmosphere. Instead, they lingered, and over decades, they drifted slowly upward into the stratosphere. Once there, exposed to the fierce, unfiltered ultraviolet light of the sun, the CFC molecules were finally shattered. This act of destruction released their chemical assassins: chlorine atoms.
A single chlorine atom, they predicted, could act as a catalyst in a long, patient demolition of the ozone layer. It would steal an oxygen atom from an ozone molecule, breaking it. Then, through another reaction, it would release that oxygen and be free to seek out another ozone molecule, and another, and another. They calculated that one chlorine atom could destroy up to 100,000 ozone molecules before it was finally neutralized.³ It was like a tiny, chemical Pac-Man, endlessly munching through our planetary shield.
When they published their findings, the work was met with skepticism and fierce resistance from the chemical industry. Could a simple, inert chemical used in refrigerators and hairspray really be unraveling the sky? For years, their theory remained just that—a dire, unproven warning
.
A Hole at the Bottom of the World
Then came Halley Bay.
When the British team finally published their data in the journal Nature in 1985, the scientific community was stunned.⁴ The “ozone hole,” as the media quickly dubbed it, wasn’t a literal hole but a region of catastrophic seasonal depletion. And it was centered over the one place on Earth where the chemical demolition was happening with terrifying efficiency: Antarctica.
Scientists soon pieced together why. During the deep, dark Antarctic winter, a swirling vortex of winds traps a mass of super-chilled air over the continent. In this frigid environment, wispy, iridescent clouds form high in the stratosphere. These Polar Stratospheric Clouds, beautiful to behold, provided a perfect microscopic surface for chlorine chemistry to run wild. All winter long, inert chlorine compounds collected on these ice crystals. When the sun finally returned in the spring, its light triggered the chemical reactions that unleashed the chlorine atoms all at once.
The Antarctic ozone hole was the smoking gun. It proved Rowland and Molina’s hypothesis with terrifying clarity. The public was galvanized by images of a gaping wound in our atmosphere. The sky, it seemed, was indeed falling.
A Treaty to Heal the World
What happened next is perhaps the most remarkable part of the story. Faced with an undeniable existential threat, the world acted. Science, industry, and policy makers mobilized with a speed and unity that remains stunning to this day.
In 1987, just two years after the hole was confirmed, 46 countries gathered to sign the Montreal Protocol on Substances that Deplete the Ozone Layer. It wasn’t a vague promise; it was a binding, ambitious global treaty to phase out the production and use of CFCs and other ozone-destroying chemicals.
The Montreal Protocol succeeded where so many other international efforts fail for a few key reasons. It was decisive, setting clear, legally-binding targets. It was fair, creating a fund to help developing nations transition to safer alternatives. And most importantly, it was adaptable. It was designed as a “start and strengthen” agreement, allowing its restrictions to be tightened as more scientific evidence became available, which it was, multiple times.⁵ It stands today as the only UN treaty in history to be ratified by every single country on Earth.
Did You Know? The Scent of a Storm
Long before we understood its chemistry, humans knew ozone by its smell. The word ozone comes from the Greek ozein, meaning “to smell.” It’s the sharp, clean, strangely metallic scent you can sometimes detect in the air after a powerful thunderstorm. That smell is ozone being created by the immense electrical energy of a lightning strike, which splits atmospheric oxygen molecules (O₂) apart, allowing them to reform as O₃. For a time in the late 19th century, before its true nature was known, this “scent of lightning” was even thought to be a healthy, purifying substance.
The Long Mend
The Montreal Protocol worked. Almost immediately, the amount of chlorine being pumped into the atmosphere began to fall. But the sky is a patient patient. The CFCs already up there would take decades to dissipate, and the healing would be a slow, generational process.
Today, we can see the results. According to the latest assessments from NASA and the United Nations, the ozone layer is steadily recovering.⁶ The hole over Antarctica still forms each spring, but it is smaller and less severe than it was at its peak in 2000. Projections show that the ozone layer over the mid-latitudes should recover to 1980 levels by around 2040. The stubborn Antarctic hole is expected to be fully closed by 2066.
The story continues to evolve. In 2016, the protocol was updated with the Kigali Amendment. This update targets hydrofluorocarbons (HFCs), the chemicals developed to replace CFCs. While safe for the ozone layer, HFCs were discovered to be potent greenhouse gases, thousands of times more powerful than carbon dioxide. By phasing them down, the Montreal Protocol is now also one of our most effective tools in the fight against climate change.⁷
A Blueprint for Hope
The rescue of the ozone layer is more than a triumphant chapter in environmental history. It is a blueprint for hope. It is a shining example of what humanity can achieve when faced with a global crisis. It proves that when scientific warnings are heeded, when nations are willing to cooperate, and when industry is spurred to innovate, we are capable of solving even the most daunting environmental challenges.
In an era dominated by the seemingly intractable problem of climate change, the story of the ozone hole serves as a powerful reminder. We have healed the sky once before. We can do it again.
Bibliography & For Further Reading
Farman, J. C., Gardiner, B. G., & Shanklin, J. D. (1985). Large losses of total ozone in Antarctica reveal seasonal ClOx/NOx interaction. Nature, 315(6016), 207-210.
Molina, M. J., & Rowland, F. S. (1974). Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. Nature, 249(5460), 810-812.
NASA Ozone Watch. "Ozone Basics." https://ozonewatch.gsfc.nasa.gov/facts/basics_en.html
Solomon, S. (1990). Progress towards a quantitative understanding of Antarctic ozone depletion. Nature, 347(6291), 347-354.
United Nations Environment Programme. "About the Montreal Protocol." https://www.unep.org/ozonaction/who-we-are/about-montreal-protocol
World Meteorological Organization (WMO). (2022). Scientific Assessment of Ozone Depletion: 2022.
United Nations Environment Programme. "Kigali Amendment." https://www.unep.org/ozonaction/kigali-amendment