How Synthetic Ammonia Changed Farming and Shaped the Modern World
Nearly bread from thin air?
1. Alchemy at Midnight
It’s nearly midnight in Karlsruhe Germany, 1909. In a cramped, ill-lit laboratory, chemist Fritz Haber wipes sweat from his brow and watches a gleaming droplet condense inside his jury-rigged high-pressure reactor. He’s been at this for days, mixing air and hydrogen, tinkering with catalyst recipes, risking explosion with every attempt. And suddenly, against a century of failed hopes, there it is. One tiny bead of ammonia, a simple molecule, but the key to feeding a hungry world¹.
2. Introduction: The Most Useful Molecule You Never Think About
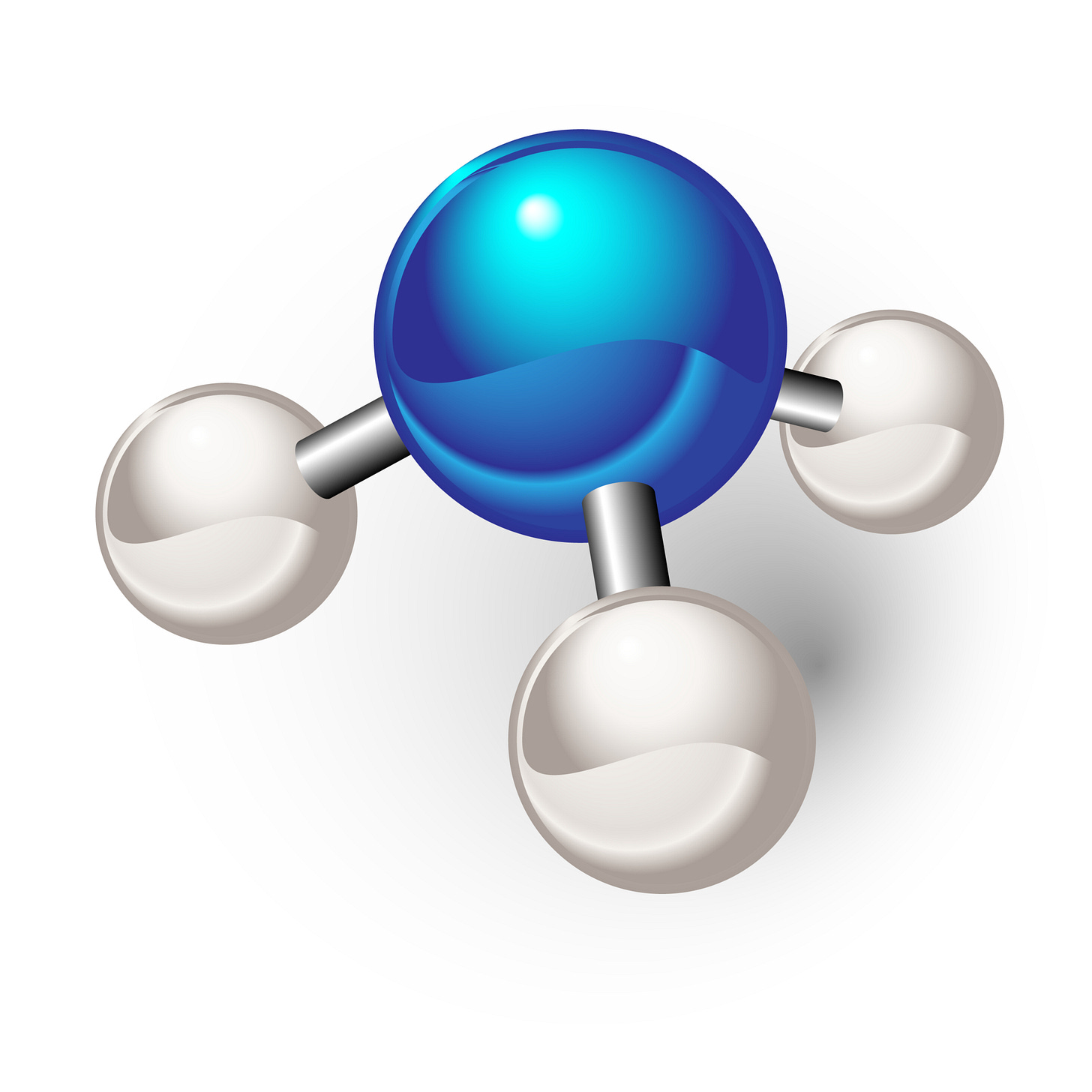
What is ammonia? For most of us, it’s the tangy smell in glass cleaner or the harsh reek from an old mop bucket. Chemically, ammonia (NH₃) is just three hydrogens and a lone nitrogen, a humble cluster of atoms, invisible yet essential for life itself.
Every leaf, loaf of bread, and living being depends on nitrogen. Plants crave it to make proteins; animals crave plants. But atmospheric nitrogen, which makes up 78% of the air, is locked away as triple-bonded N₂ gas, nearly impossible for living things to use². Before 1910, the world’s farms ran on guano (bat droppings), Chilean saltpeter, and the errant bolt of lightning—each one a rare, almost miraculous source of plant-friendly nitrogen.
Yet, by the dawn of the 20th century, the world faced a slow-motion catastrophe: the Great Nitrate Crisis. Populations were booming, soils were hungry, and old supplies were running out. Some projected imminent mass famine, as growth outstripped nature’s ability to feed us³.
3. The Nitrogen Dilemma
Nitrogen is both generous and stingy, everywhere but inaccessible. Only a few living organisms (notably certain bacteria) could snatch it from the air and “fix” it into forms plants could eat. Everyone else? They scoured the earth for guano islands, nitrate mines, or, in wartime, desperate chemical tricks.
In the mid-1800s, imperial powers sent navies and laborers to harvest Andean guano hills, fueling both bread baskets and battlefields. Even empires risked war for bags of poop —a fact almost too absurd for fiction. But by 1900, these sources were failing. If no solution came, would human civilization hit its fundamental ceiling?
4. Lighting the Spark: How Haber and Bosch Conquered Air
Fritz Haber was obsessed. For years, chemists had battered their heads against the puzzle of nitrogen, coaxing it, bombarding it, but always falling short. Haber, fueled by doggedness and ego, tried something new: combine air (nitrogen) and hydrogen under enormous pressure and heat, then pass it all over a metal catalyst (at first, rare osmium; soon, abundant iron worked almost as well). If luck held, NH₃—ammonia—would form⁴.
That June night in 1909, as he and his assistant watched ammonia droplets form, they realized they’d cracked the code. But making a few milliliters in a lab was a far cry from feeding nations.
That’s where Carl Bosch came in—a chemical engineer with a mind for machinery and a faith in the possible. Bosch’s challenge: scale up Haber’s delicate trick to a roaring factory, able to withstand steel-bending pressures and channel rivers of gas. After bruising setbacks (valves melted, alloys failed, entire plants stalled), the joint Basf (Badische Anilin- und Sodafabrik) team triumphed. In 1913, the first Haber-Bosch plant in Oppau, Germany, began turning literal air into gold—pouring out ammonia by the ton⁵.
5. From Air to Bread: A New World Unfolds
Ammonia itself is pungent and unlovely, but in the right hands it’s magic. Just add oxygen, and it becomes nitrate—the backbone of commercial fertilizer.
With synthetic ammonia, farmers everywhere could break free of the ancient chains: no need for guano, no Chilean mines. Fields bloomed with abundance. Wheat stalks grew lush and thick, rice paddies yielded bumper harvests, and the grip of famine began to loosen.
The numbers stagger. Today, over half the people alive are fed by crops grown with Haber-Bosch fertilizer⁶. If you pulled the ammonia plug, half the world would go hungry.
This molecular wizardry changed everything—from diets to demographics, geopolitics to the arc of everyday life. Humanity roared forward, fueled by a molecule once beyond its grasp.
6. Bombs and Backlashes: The Double-Edged Sword
But no magic comes without shadows. In World War I, Germany’s British blockade threatened to starve its explosives factories of nitrate. Enter Haber-Bosch: not only did it feed crops, it kept munitions lines banging out shells. Although perhaps grim, ammonia’s legacy is both loaf and bullet.
As the Green Revolution unfolded, another, subtler crisis loomed. Synthetic fertilizer runoff seeped into rivers, fueling algae blooms and creating “dead zones” in oceans where nothing larger than bacteria could survive⁷. The altered nitrogen cycle brought richer harvests—at the price of destabilized climates and poisoned waterways.
Humanity became addicted. Today, over 170 million metric tons of ammonia cascade through global agriculture, chemical plants, and fuel systems every year⁸. Even as we confront climate change, rising populations, and fractured ecosystems, we cannot turn away.
7. Quirks and Curiosities: Did You Know?
The “Bread from Air” Miracle: When Haber’s process debuted, German newspapers swooned over the concept of “Brot aus Luft”—bread conjured from thin air⁹.
Unintended Consequences: Fritz Haber, hailed for feeding billions, also pioneered chemical warfare—developing chlorine gas for the trenches.
Ammonia in Space: NH₃ isn’t just a terrestrial player; vast lakes of it swirl on Jupiter and Saturn. On Saturn’s moon Titan, ammonia may help shape extraterrestrial chemistry10.
Haber’s Dilemma: Awarded the Nobel Prize in Chemistry (1918) for “synthesizing bread from air,” Haber’s legacy remains fraught—a hero to some, a villain to others.
8. Today’s Reckoning: Can We Green the Miracle?
As the 21st century unfurls, the very tools that saved billions now threaten to undermine us. Ammonia factories consume immense energy, much from fossil fuels; fertilizer overuse grinds down soils and surges into waterways. The nitrogen cycle, once a slow dance, now stampedes.
Yet, hope stirs. Scientists race to engineer “green ammonia” using wind- or solar-powered electrolysis, striving to sever ammonia’s dependency on natural gas. Others engineer crops that host their own nitrogen-fixing bacteria, hinting at a future where fields fertilize themselves¹¹.
The question now: can we keep the bounty without courting disaster? Or, as with all great inventions, does every solution simply “kick the can” down the road?
9. Conclusion: The Fate of a Molecule, The Shape of Tomorrow
From Andean guano fields to the echo of ammonia reactors, humanity’s fate remains entwined with the chemistry of nitrogen. The Haber-Bosch process did more than unlock bread from air—it unshackled a limitation on civilization, for better and for worse.
Will we invent a new balance, or will Haber’s boon cost us yet? The story of ammonia is far from over—a flicker at the edge of discovery, history, and the world to come.
10. For Further Reading
The Alchemy of Air by Thomas Hager (Amazon link)
Enriching the Earth by Vaclav Smil (Publisher link)
National Geographic: Why Ammonia Is the Most Important Industrial Chemical
Nature: The Haber–Bosch Reaction: the Engine of the Modern World